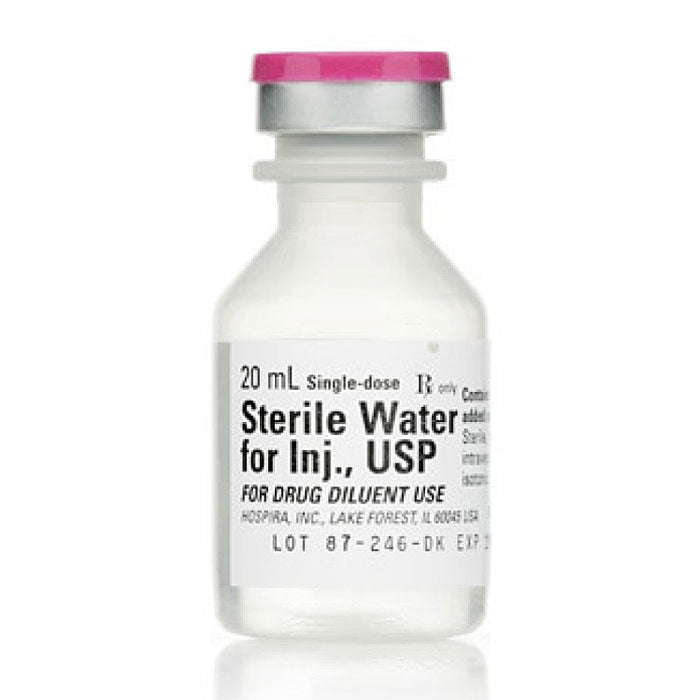
Sterile Water for Injection 20ml, tray of 25 (Rx)
(Note: We don’t Fill Personal Prescriptions)
How to Order:
This preparation is designed solely for parenteral use only after addition to drugs that require dilution or must be dissolved in an aqueous vehicle prior to injection.
Sterile Water for Injection contains no bacteriostat, antimicrobial agent or added buffer and is supplied only in single dose containers to dilute or dissolve drugs for injection. For IV injection, add sufficient amount to a solute to make an approximately isotonic solution. pH 5.0 to 7.0.
Water for Injection, is chemically designated H2O.
Please note this item is not Bateriostatic and does not contain Benzyl Alcohol. If that is needed please see our Bacteriostatic Water page.
What is the difference between Sterile Water and Bacteriostatic Water?
Bacteriostatic water for injection has an extra preservative agent added into the water called benzyl alcohol (BnOH). Benzyl alcohol is a colorless liquid that has low toxicity, and low vapor pressure. Sterile Water for injection is a single-dose sterile water that does not contain any bacteriostatic, antimicrobial agents, and added buffers. Purchase online today as they are extremely hard to find in the United States.
Storage: Store at 20 to 25 degrees Celsius (68 to 77 degrees Fahrenheit).