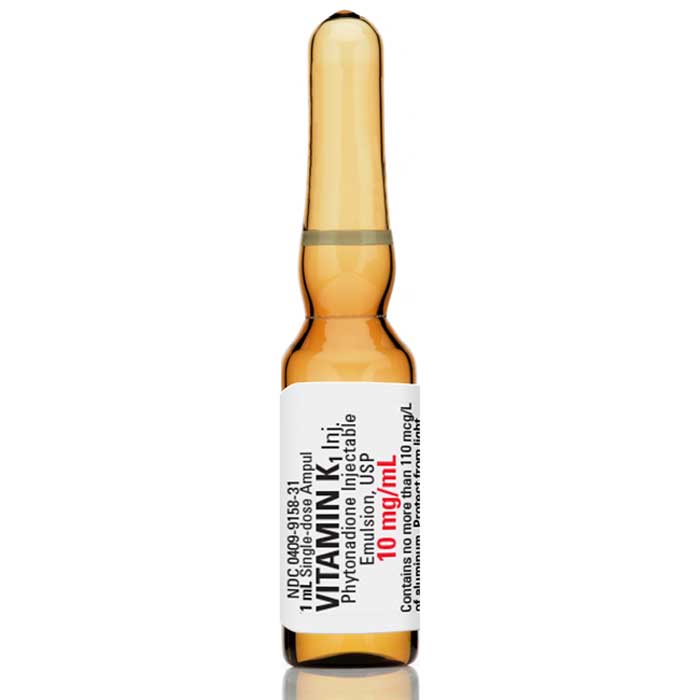
Vitamin K1 Injection (Phytonadione Injectable Emulsion) 10 mg/ mL by Pfizer 25/Pack
(Note: We don’t Fill Personal Prescriptions)
How to Order:
Pfizer Vitamin K1 Injection (Phytonadione Injectable Emulsion) 1 mg/0.5 mL Neonatal is a prescription medication used to prevent and treat vitamin K deficiency in newborn babies. It is administered by injection and contains a synthetic form of vitamin K1, which helps the body to form blood clots and prevent bleeding. This injection may be recommended for babies who are at risk of vitamin K deficiency due to complications during delivery or certain medical conditions. It is often given to newborn babies shortly after birth as a routine preventative measure.
Vitamin K1 Injection (Phytonadione Injectable Emulsion, USP) aqueous dispersion of vitamin K1 for parenteral injection, possesses the same type and degree of activity as does naturally-occurring vitamin K, which is necessary for the production via the liver of active prothrombin (factor II), proconvertin (factor VII), plasma thromboplastin component (factor IX), and Stuart factor (factor X). The prothrombin test is sensitive to the levels of three of these four factors−II, VII, and X. Vitamin K is an essential cofactor for a microsomal enzyme that catalyzes the post-translational carboxylation of multiple, specific, peptide-bound glutamic acid residues in inactive hepatic precursors of factors II, VII, IX, and X. The resulting gamma-carboxy-glutamic acid residues convert the precursors into active coagulation factors that are subsequently secreted by liver cells into the blood.
Phytonadione is readily absorbed following intramuscular administration. After absorption, phytonadione is initially concentrated in the liver, but the concentration declines rapidly. Very little vitamin K accumulates in tissues. Little is known about the metabolic fate of vitamin K. Almost no free unmetabolized vitamin K appears in bile or urine.
In normal animals and humans, phytonadione is virtually devoid of pharmacodynamic activity. However, in animals and humans deficient in vitamin K, the pharmacological action of vitamin K is related to its normal physiological function, that is, to promote the hepatic biosynthesis of vitamin K dependent clotting factors.
The action of the aqueous dispersion, when administered intravenously, is generally detectable within an hour or two and hemorrhage is usually controlled within 3 to 6 hours. A normal prothrombin level may often be obtained in 12 to 14 hours.
In the prophylaxis and treatment of hemorrhagic disease of the newborn, phytonadione has demonstrated a greater margin of safety than that of the water-soluble vitamin K analogues.
Indications and Usage
Vitamin K1 Injection (Phytonadione Injectable Emulsion, USP) is indicated in the following coagulation disorders which are due to faulty formation of factors II, VII, IX and X when caused by vitamin K deficiency or interference with vitamin K activity.
Vitamin K1 Injection is indicated in:anticoagulant-induced prothrombin deficiency caused by coumarin or indanedione derivatives; prophylaxis and therapy of hemorrhagic disease of the newborn; hypoprothrombinemia due to antibacterial therapy;
hypoprothrombinemia secondary to factors limiting absorption or synthesis of vitamin K, e.g., obstructive jaundice, biliary fistula, sprue, ulcerative colitis, celiac disease, intestinal resection, cystic fibrosis of the pancreas, and regional enteritis; other drug-induced hypoprothrombinemia where it is definitely shown that the result is due to interference with vitamin K metabolism, e.g., salicylates.