Injectafer (Ferric Carboxymaltose) Injection 75mg/15mL Vial
(Note: We don’t Fill Personal Prescriptions)
How to Order:
Injectafer (Ferric Carboxymaltose) Injection 75mg/15mL Vial is a prescription iron replacement medication used to treat iron deficiency anemia in adults who are unable to take oral iron supplements or whose anemia has not responded to oral iron treatments. This injection contains ferric carboxymaltose, a form of iron that is easily absorbed by the body and can help increase your red blood cell count. It is administered by a healthcare professional via injection into a vein (intravenous or IV) and may require multiple doses to adequately address iron deficiency anemia
Injectafer (ferric carboxymaltose) injection is used to treat iron deficiency anemia in adult patients who have intolerance to oral iron or have had unsatisfactory response to oral iron, or who have non-dialysis dependent chronic kidney disease.
Ferric carboxymaltose is a form of injectable iron that is used if you cannot take iron by mouth because of side effects or an unsuccessful response to treatment. It is also used by people who have anemia due to long-term kidney disease.
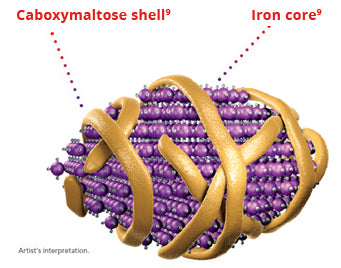
Injectafer is a dextran-free formulation of colloidal iron (III) hydroxide in complex with carboxymaltose, a carbohydrate polymer that releases iron.
- The chemical characteristic of the iron-carbohydrate complex means that iron is released slowly, avoiding toxicity and oxidative stress Iron infusion carboxymaltose molecule.
Directions and Usage:
For patients weighing 50 kg (110lb) or more: Give Injectafer in two doses separated by at least 7 days. Give each dose as 750 mg for a total cumulative dose not to exceed 1500 mg of iron per course. For patients weighing less than 50 kg (110lb): Give Injectafer in two doses
separated by at least 7 days. Give each dose as 15 mg/kg body weight for a total cumulative dose not to exceed 1500 mg of iron per course. The dosage of Injectafer is expressed in mg of elemental iron. Each mL of Injectafer contains 50 mg of elemental iron. Injectafer treatment may be
repeated if iron deficiency anemia reoccurs. Administer Injectafer intravenously, either as an undiluted slow intravenous push or by infusion. When administering as a slow intravenous push, give at the rate of approximately 100 mg (2 mL) per minute. When administered via
infusion, dilute up to 750 mg of iron in no more than 250 mL of sterile 0.9% sodium chloride injection, USP, such that the concentration of the infusion is not less than 2 mg of iron per mL and administer over at least 15 minutes. When added to an infusion bag containing 0.9% sodium chloride injection, USP, at concentrations ranging from 2 mg to 4 mg of iron per mL, Injectafer
solution is physically and chemically stable for 72 hours when stored at room temperature. To maintain stability, do not dilute to concentrations less than 2 mg iron/mL. Inspect parenteral drug products visually for the absence of particulate matter and discoloration prior to administration. The product contains no
preservatives. Each vial of Injectafer is intended for single-use only. Any unused drug remaining after injection must be discarded. Avoid extravasation of Injectafer since brown discoloration of the extravasation site may be long lasting. Monitor for extravasation. If extravasation occurs, discontinue the Injectafer administration at that site.
Brand: Gensiasico
Size: 750 mg iron / 15 mL single-use vial
NDC: 00517-0650-02
UPC: 305170650025
Purpose: Iron Deficiency Treatment
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions: Serious hypersensitivity reactions, including anaphylactic-type reactions, some of which have been life-threatening and fatal, have been reported in patients receiving Injectafer. Patients may present with shock, clinically
significant hypotension, loss of consciousness, and/or collapse. Monitor patients for signs and symptoms of hypersensitivity during and after Injectafer administration for at least 30 minutes and until clinically stable following completion of the infusion. Only administer Injectafer when personnel and therapies are immediately available for the treatment of serious hypersensitivity reactions.
Buy Injectafer (Ferric Carboxymaltose) Injection to online at Mountainside Medical Equipment.